Introduction
Aristotle believed that the matter in the universe was made of four elements:
earth, air, fire and water that were subjected to two forces:
- Gravity, which made earth and water "sink".
- Levity, which made air and fire to "rise".
At Aristotle's time the matter was assumed to be "continuous", that it could be divided in smaller and smaller pieces indefinitely. However, some Greek physicists -for instance, Democritus- already thought that the matter was ultimately made of a large number of some kinds of "grains" -or atoms- who could not be divided any further.
Nothing changed much until the British chemist and physicist John Dalton noticed in 1803 that chemicals always combined in definite proportions. This could only be explained if matter was made of atoms that combined together to form molecules. But still not all scientists agreed.
In 1905, before he wrote his theory of special relativity, Einstein had published another paper about Brownian motion, the random motion of particles of dust immerged in a liquid. In it, Einstein showed that this phenomenon could only be explained by the interaction of the atoms of the liquid with the dust particles. The existence of what was then considered by many as the ultimate indivisible part of the matter, the atoms, had been confirmed.
However, a few years before, another British physicist, J.J. Thompson, had shown experimentally that there were small particles of matter, negatively charged, called electrons. Their mass was less than one thousandth the mass of the lightest atom (hydrogen). Another British scientist, Ernest Rutherford, showed in 1911 that the atoms are not the ultimate pieces of the matter. Rutherford showed that they are composed of a small nucleus positively charged around which some electrons are orbiting.
Initially it was thought that the nucleus of the atom was made of negatively charged electrons and by a higher number of positively charged particles that were called protons. It took until 1932 for James Chadwick -still another British scientist- to show that the nucleus of the atom was composed instead of a number of positively charged protons -exactly the same number than the number of electrons orbiting the nucleus- and by a new type of particles called neutrons. The neutrons have practically the same mass as the protons, but they have no electrical charge.
Until about 1965 scientists thought that the neutrons and the protons were
elementary indivisible particles. At that time, new sophisticated experiments
involving accelerating protons at very high speed to make them collide with
other protons, or with electrons, showed that they were made up of smaller
particles. They were called "quarks" by their discoverer, the
American Murray Gell-Mann. Scientists now believe that there are six types
of quarks named: UP, DOWN, STRANGE, CHARMED, BOTTOM and TOP. Moreover all
these six types of quarks come in three colours: red, green and blue (but
these names are no more than labels, they have no colour as they are smaller
that the wavelengths of light). As a result the substructure of the atoms
can be described as follow:
- A proton is made of three quarks, one of each colour, two UP quarks and
one DOWN quark. A neutron is also made of three quarks, one of each colour,
but there are now two DOWN quarks and one UP.
- Scientists created other particles made from the other quarks (STRANGE,
CHARMED, BOTTOM and UP). These have a greater mass and they quickly decay
into neutrons and protons.
Properties of Electrons, protons, neutrons and their sub-particles
As we have seen the atoms, the protons and the neutrons are not the ultimate particles. So, what are these elementary bits of matter? Since the wavelength of visible light is longer that an atom, we cannot "see" the atoms' components this way. But elementary particles also behave like waves with wavelengths decreasing with their energy -or velocity- and, as we can accelerate them at very high speed in modern accelerators -up to a few thousands of millions of electron volts- this is the way to detect them. The experimental physicists showed that atoms, protons, and neutrons are made of smaller particles. As the accelerators accelerate particles to higher and higher speeds -or energy- perhaps one will find that what we now consider as elementary particles are in fact composed of even smaller ones, although the actual theories show that we have reached, or at least that we are close, to the ultimate basic components of the matter.
Everything in nature, including light and gravity, behave like particles,
and particles have a property called "Spin." Spin tells us how
particles look from every direction:
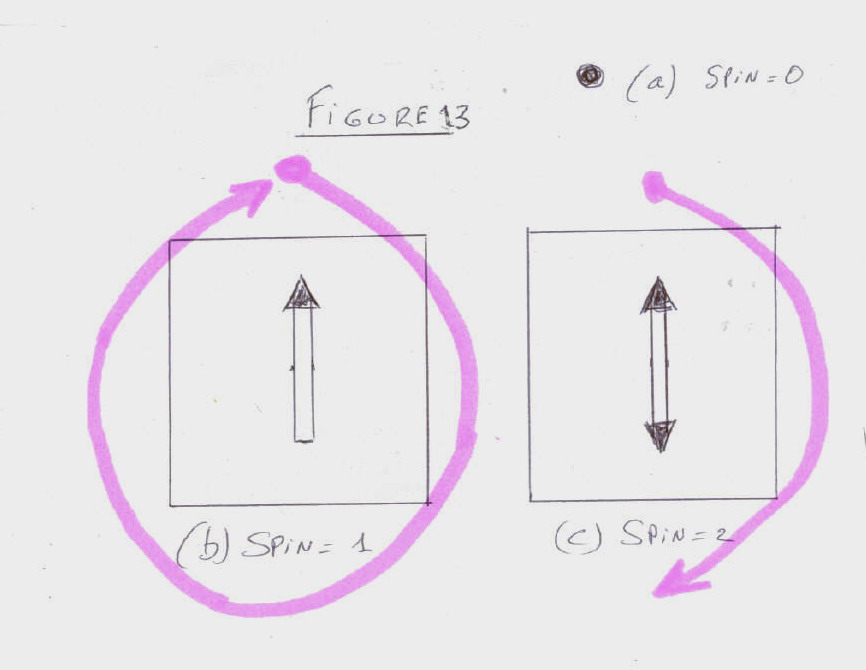
- A particle of spin 0 in like a dot, it looks the same from all direction
(Figure 13a).
- A particle of spin 1 looks like a single arrow. One must turn it 360 degrees
to make it looks the same (Figure 13b).
- A particle of spin 2 is like a double arrow. One only has to turn it 180
degrees to make it looks alike (Figure 13c).
- A particle with spin ½ must be turned twice 360 degrees to look
the same.
- There are also particle of spin higher than 2. They look alike if one
turn them a small fraction of 360degrees.
All the particles can be divided into two groups:
- The particles of spin ½ make all the matter in the universe. They
follow the exclusion principle discovered in 1925 by the Austrian physicist
Wolfgang Pauli. According to this, two similar particles cannot exist in
the same state; they cannot have the same position and velocity within the
limits of the uncertainty principle.
- The particles of spin 0, 1 and 2 are the particles that create the forces
linking the matter particles together.
The exclusion principle, among other things, explains why the particles do not collapse on themselves in a state of high density under the effects of the forces of spin 0, 1 and 2. As we have seen, according to the exclusion principle, if the particles come very close to each other they must have different velocities and they will soon move to another position. Without the effect described by this principle, elementary particles would collapse on each other in a uniform state of high density.
In 1928 Dirac finally explained why electrons had a ½ spin and why one must rotate them twice 360 degrees to have them looking the same again. Dirac also predicted the existence of anti-electrons -positrons- but it took until 1932 to experimentally produce some. From that time we know that every particle has an antiparticle with which it can annihilate -and generally does if they come close to each other. However, in the case of force-carrying particles, antiparticles are exactly the same as the former ones.
Does this means that there are anti-worlds and anti-persons made of antiparticles? It is better to leave the answer to philosophers but, if you meet your anti-person, do not go close to him because both of you would be annihilated.
Quantum mechanics tells us that the forces holding matter particles together
are carried by particles of spin 0, 1 or 2. This happens in the following
way:
- A matter particle -electron or quark- emits a force particle (virtual
particle).
- As a consequence the velocity of the matter particle changes.
- The force-carrying particle hit another matter particle and is absorbed.
- As a consequence, the velocity of the second matter particle is changed.
- It looks like if there had been a force between the two matter particles
that changed the velocities of both of them.
Force-carrying particles do not follow the exclusion principle. This means, among other things, that many more than one can be exchanged, and this can create very big forces. However, if the force-carrying particles have a high mass, not only they will be difficult to produce but also they will not travel long distance, and the force that they carry will have a short range. If the force-carrying particles have no mass, their action will have a long range. The force-carrying particles are said to be "virtual" particles -the matter particles being called "real"- because ordinary particle detectors cannot detect them. We know they exist through their measurable effects, such as creating forces between matter particles.
In some conditions, however, particles of spin 0, 1 and 2 can exist as real particles and they can be detected. They appear as waves such as light or gravitational waves. They may be produced when matter particles interact with each other by exchanging virtual force-carrying particles. For example, the repulsive force between two electrons is due to the exchange of virtual photons -non detectable- but, if two electrons come close to each other, they can emit real photons that are detectable as light wave.
Force carrying particles can be divided in four classes according to the
strength of the force that they carry and the particles with which they
interact.
- The gravitational force is universal in the sense that every particle
is subject to it in proportion to their mass, or energy. It is the weakest
of the four forces and, normally, we would not notice it but for two reasons.
First, the gravitational force acts over long distance and it is always
attractive. For instance, all matter particles of the sun and the earth
are subject to it and, adding up all these elementary forces, they create
a force that cannot be ignored. Secondly, in quantum mechanics language,
the gravitational force between two matter particles is carried by a particle
named graviton of spin 2. The graviton has no mass so this force has a long
range. If we go back to the example of the earth and the sun, the gravitational
force between them is due to the exchange of gravitons between each of their
particles. Gravitons are virtual particles, but their effect is real, visible,
and measurable: it makes the earth orbit the sun. Real gravitons make gravitational
waves but they have never been detected yet.
- The electromagnetic force interacts with electrically charged particles
such as protons, quarks and electrons, but not with neutral particles such
as neutrons and gravitons. This force is much stronger that the gravitational
force. The electromagnetic force between two electrons is about 10E+42 bigger
that the force of gravity between them. As the electrical charges can be
either positive or negative, the force between two positive or negative
charges is repulsive, whereas the force between two charges of different
signs is attractive. Large pieces of matter such as the earth or the sun
have more or less the same amount of positive and negative charges and the
repulsive and attractive forces cancel each other and the resulting total
electromagnetic force is close to nothing. At the level of atoms and molecules,
the electromagnetic force is the dominant one. The electromagnetic attractive
force between electrons (negative charge) and protons (positive charge)
makes the electrons orbit around the nucleus in the same way that the gravitational
force makes the earth orbit around the sun. The electromagnetic attraction
is said to be due to the exchange of a large number of virtual photons of
mass zero and of sin 1. But when an electron changes from one allowed orbit
to another closer to the nucleus it emits energy in the shape of real photons
that can be seen as visible light, if it has the right wavelength or by
photon detectors such as photographic films. Conversely if a real photon
hits an atom, one of the electrons can go on a higher orbit, the photon
looses it energy and is absorbed.
- The so-called weak nuclear force that is responsible for radioactivity
acts on all matter particles of spin ½, but not on the particles
of spin 0, 1 or 2 such as photons and gravitons. In 1967 the British Abdus
Salam and the American Steven Weinberg discovered that in addition to the
photons, there were three other spin-1 particles -known as massive vector
bosons- that carry the weak force. They are called W+, W- and Z 0, each
with a mass of about 100 GeV. According to this new theory, these particles
exhibit a property described as "spontaneous symmetry breakdown".
It means that they appear to be completely different particles at low energy,
but at high energy they are similar, although in a different state. At high
energy they behave in the same way, for instance at energy above 100GeV
the three new particles Z+, Z- and Z 0 and the photon behave in the same
way. However at lower energy, which is their normal state, the symmetry
between the particles does not exist anymore, their mass increase greatly
and the forces they carry are very short range only. These three particles
were first produced at CERN (European Centre for Nuclear Research) in Geneva
Switzerland, in 1983.
- The strong nuclear force holds the quarks together in the protons and
neutrons as well as the protons and neutrons in the atom nucleus. This force
is assumed to be carried by so-called "gluon", particles of spin
-1 that interacts only with themselves and the quarks. This strong force
has a property called confinement: it always binds particles in combinations
that have no colour. As a consequence on cannot have a quark alone because
it would have a colour; three quarks -a red, a blue and a green- are always
joined together by gluon to form neutrons or protons (mixing the red, blue
and green colours gives white). The only other possibility is for a quark
(for instance a red one; but it is also true for the green and the blue
quarks) to be joined to an anti-quark of anti red colour to form mesons.
These mesons are unstable because the quark and its anti-quark can, and
will, annihilate each former to form an electron and other particles. Confinement
also prevents to have a gluon on its own because gluons too have colour.
They must join together so that their combined colour is white, to form
an unstable particle called a glueball. Another property of the strong nuclear
force is called asymptotic freedom. At normal energy, the nuclear force
is very strong but it has been shown experimentally that at high energy
the nuclear force becomes much weaker, allowing the quarks and gluons to
behave more or less like free particles.
Facts and Theories on how the matter is made
The interference between particles (see part 7-Quantum mechanics) is at the base of the way we now understand the structure of the atoms. Until the beginning of the 20th century, scientists thought that atoms were like the planets orbiting the sun in the solar system. In the atoms it was believed that electrically negative charged electrons were orbiting the positive charged central nucleus. The attraction between these positive and negative components of the atoms was assumed to keep the electrons on their orbit in the same way that gravity keeps the planets stable on their orbits. But the laws of electricity and mechanics predicted that the electrons would loose energy and gradually collapse on the nucleus. This meant that all the matter in the universe would finally collapse on itself creating a "singularity" of high density.
In 1913 the Danish scientist Niels Bohr suggested that the electrons were not able to move on all orbit around the nucleus, but only on orbits at well-defined distances from it. If, moreover, one assumes that only one or two electrons can move on each of these specific orbits, it would explain why they do not collapse on the nucleus. According to Bohr's theory, the electrons cannot move closer to the nucleus once all the orbits closer to the nucleus, starting from the smaller one, are filled according to Bohr's law. This theory explained quite well the structure of the hydrogen atom, the one that has only one electron. However, this law did not seem applicable to atoms with more electrons.
At that time the suggestion that electrons could only move on well-defined orbits seemed arbitrary. Quantum mechanics, finally, gave a logical explanation to this phenomenon. According to Quantum mechanics, electrons orbiting around a nucleus can be viewed as particles as well as waves, with a wavelength proportional to their velocity. The orbits that have a circumference equal to a whole number of wavelengths, and only these ones, are the "possible" orbits as defined by Bohr. In all the other orbits the wave crests would eventually cancel each other. Quantum mechanics allows scientists to calculate the possible electron orbits of the atoms with more than one electron, and also in molecules (nuclei made up of more than one atom held together by the rotating electrons orbiting more than one nucleus). Quantum mechanics allows the scientists to calculate, in principle, everything that exists in the natural world -in particular in chemistry and biology-, but within the limits of the Uncertainty Principle. However, when there are more than a few electrons around a nucleus, the calculations are so complicated that on cannot do them.
On the base of the successful unification of the electromagnetic and weak nuclear forces scientists wanted to go one step further, and device a so-called Grand Unified Theory (GUT) that would also include the strong nuclear forces. Gravity forces would still not be included limiting the scope of GUT, but it would still be a big step towards a completely unified theory that would also include gravity in the future. Einstein worked most of his life towards this aim but even him, to his great displeasure, did not succeed.
It is known that the strong nuclear forces become weaker at high energies, while the electromagnetic and weak nuclear forces become stronger when their energies is increased. At very high energy -above what is called the Grand Unification Energy- these three forces would have the same strength, and could then be thought to be three different aspects of the same force. Moreover, above that energy, the matter particles with ½ spin -quarks and electrons- would become the same, another unification. The value of the Grand Unification Energy is not known with accuracy, but it is believed to be at least 10E+15 GeV. The accelerators that will be built in the near future will accelerate particles to energies of a few thousands GeV, far away the value of the grand unification energy. It fact, to be able to accelerate particles at this level of energy, accelerators would have to be as big as the solar system, and this is unthinkable. Fortunately there are low-energy effects of this theory that can be tested, like protons decaying in lighter particles such as anti-electrons. Although experiments have been made, the probability of such transformations is so low that none has not been observed yet.
It is possible to imagine the reverse process, the production of protons and neutrons, or better quarks -the components of the matter in our universe- like at a time when there were no more quarks than anti-quarks. This would explain how the universe began, or was created. As we know there are no antiprotons or antineutrons in our universe with the exception of those scientists created in accelerators. This is also the case in our galaxy. However, it is not so clear if other galaxies are made up of protons and neutrons, or of antiprotons and antineutrons. All we know is that it cannot be a mixture of both because, even there, matter and antimatter would annihilate each other creating strong radiations. At the present time scientists believe that the other galaxies are made of matter, and not antimatter.
The Grand Unified Theory (GUT) explains why the universe contains more quarks than anti-quarks, although it started with an equal number of both. GUT tells us that quarks can decay into anti-electrons at high energy. But it also allows anti-quarks to change into electrons, as well as electrons and anti-electrons changing into quarks and anti-quarks. This only happened naturally at the beginning of the universe when the temperature was high enough. The fact that there are now more quarks than anti-quarks is due to the fact that the laws of physics are not the same for these two particles.
Until the 1950s it was believed that the laws of physics were subject to
three symmetries:
- The symmetry C meant that the laws of physics are the same for particles
and antiparticles.
- The symmetry P states that the laws are the same for any situation and
its mirror image (the mirror of a particle spinning in one direction is
spinning in the other).
- In the symmetry T, if one reverses the direction of motion of all particles
and antiparticles, the system goes back as it was before. That means that
the laws of physics are the same in the forward and backward direction of
time.
In 1956 the American physicists Tsung-Dao and Chen Ning Yang suggested that the weak nuclear forces do not follow the symmetry P. In consequence, due to the weak forces' actions, our universe and its mirror image would have developed in different ways. Soon after, it was proved that this theory was right. The weak forces do not follow the symmetry C either (this would make an universe composed of antiparticles to behave differently from ours). But the weak forces follow the symmetry CP, and this means that our universe and its mirror image would develop the same way if every particle was replaced by its antiparticle. However, there are exceptions to this rule. Decaying K-mesons do not follow the CP symmetry.
A recent theory states that all laws that obey quantum mechanics and relativity
must obey the CPT symmetry. That is, the universe would behave the same
if:
- Particles are replaced by antiparticles.
- One takes the mirror image.
- One reverses the direction of time.
It was then proved that if one only changes the particles into anti-particles and takes the mirror image without reversing the direction of time, the universe would not behave the same way. The laws of physics change if one reverses the direction of time because they do not obey the T symmetry.
It is certain that at the beginning the universe did not obey the T symmetry since as time goes forward the universe expands, and if the time ran backward it would contract. From this it follows that as some forces do not obey the T symmetry and as the universe expands, more anti-electrons turn into quarks that electrons into anti-quarks. As the universe expands and cools down, quarks and anti-quarks annihilate each other but, as there are more quarks, the resulting universe is made of what we call matter, and not antimatter. It must be clear that the notion of quarks and anti-quarks is not clear. If, for example, there had been an excess of anti-quarks after the universe expanded and cooled down, we would have called anti-quarks quarks and inversely.
The Grand Unified Theory does not include gravity but, as it is a weak
force, it does not really make a difference when we are dealing with particles
or atoms. However, as gravity is a long-range always-attractive force, it
becomes important when dealing with big bodies. This explains why gravity
is the main factor when dealing with the universe. It is, for instance,
fully responsible for the collapse of some stars and the formation of black
holes.